
Proper Thru-Hull Bonding |
 |
 |
|
The first picture (above left) shows a method of bonding all thru-hulls that are eventually connected with the engine ground in order to prevent galvanic corrosion and
possible fitting failure that may lead to flooding or even a vessel total loss. Do not think or believe (from various other sources) that because you have a fiberglass vessel
you are immune (see bottom picture) from this problematic situation. The various metals (brass, bronze, steel, ...) that make up the thru-hulls and attached fittings are in
essence creating a small battery due to the fact you have many different metallic compounds immersed in salt water. Multiply this by the many thru-hulls installed. Over time,
the induced corrosion will erode the metal away unless it is bonded. Correct vessel bonding is overlooked too many times until extensive damage occurs and is realized. The
cost of repair is much more significant than ensuring you have a properly bonded vessel. Contact ZRD for full details.
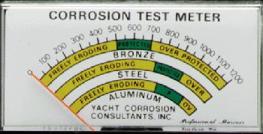
The second picture (above right) shows the front panel face of a dedicated galvanic meter with the scales that apply (using its specific reference electrode) to various metal types and the readings that would give protection.
You may use a standard inexpensive electronic meter (shown in the pictures above) if you have access to or purchase a Silver/Silver Chloride probe (below left picture). Attach one end of the probe to the positive connection on the meter and the other end is placed in the sea (salt) water. The negative lead from the meter is connected to the item being checked. If the connections are reversed, the reading will be negative (as some prefer to see it displayed), but the absolute value is what is needed to determine the level of protection. Using a GUEST Model 2435 Ag/AgCl sensor, its typical values are provided in a table (below right table) as a guide.
Depending on the specific reference electrode used, typical voltage values are provided above. Each underwater metal is protected from corrosion when the protection system is able to induce and maintain a negative shift (raise metal's potential value) of at least 0.20V above its freely existing (non-protected) value. An additional shift results in overprotection and may cause paint to peel from a metal hull, decreased effectiveness of anti-fouling paints and barrier coatings on steel or fiberglass hulls, or chemical damage to a wooden hull. Technical Discussion/Description
Galvanic corrosion occurs when two dissimilar metals (Galvanic Series below) are connected together electrically in the presence of a conductive electrolyte. An electrical potential is generated. Atoms in the less noble metal give up their electrons to the more noble metal and are released to flow (corrode) into the electrolyte in the form of positively charged ions. If a voltmeter is placed in the circuit, it will measure the potential between the anode and cathode of the cell. This will provide a measurement of the tendency to corrode. Active metals like zinc are commonly used to protect more noble metals. If the two metals are immersed in the same electrolyte and deliberately connected by an electrical "bonding" system, the zinc will give up it's electrons to protect the more noble metal. In this configuration, the zinc is called the anode and the protected metal is called the cathode. For testing purposes, the item being tested acts as one half of the cell and the other half is the reference half-cell. The silver-silver chloride half-cell consists of silver immersed in a solution of silver chloride, which is in contact with sea water via a porous plug. If the item being tested is corroding, positive ions will be in solution leaving behind electrons in that item. When the half-cell is placed into this area of positive ions, the distribution of ions inside the porous plug are polarized, in such a way that, silver ions migrate to the silver rod in the middle, and chloride ions migrate towards the porous membrane. This creates an overall positive charge in the immediate vicinity of the silver rod. Since the half-cell is connected, a galvanic cell is again set up. A voltmeter is connected to measure the potential differenece between them. The potential of the item with respect to the half-cell is always quoted as a negative value. |
|
CORRODED END (ANODIC OR LEAST NOBLE)
MAGNESIUM
MAGNESIUM ALLOYS ZINC ALUMINUM 5052, 3004, 3003, 1100, 6053 CADMIUM ALUMINUM 2117, 2017, 2024 MILD STEEL (1018), WROUGHT IRON CAST IRON, LOW ALLOY HIGH STRENGTH STEEL CHROME IRON (ACTIVE) STAINLESS STEEL, 430 SERIES (ACTIVE) 302, 303, 321, 347, 410,416, STAINLESS STEEL (ACTIVE) NI - RESIST 316, 317, STAINLESS STEEL (ACTIVE) CARPENTER 20CB-3 STAINLESS (ACTIVE) ALUMINUM BRONZE (CA 687) HASTELLOY C (ACTIVE) INCONEL 625 (ACTIVE) TITANIUM (ACTIVE) LEAD - TIN SOLDERS LEAD TIN INCONEL 600 (ACTIVE) NICKEL (ACTIVE) 60 NI-15 CR (ACTIVE) 80 NI-20 CR (ACTIVE) HASTELLOY B (ACTIVE) BRASSES COPPER (CA102) MANGANESE BRONZE (CA 675), TIN BRONZE (CA903, 905) SILICONE BRONZE NICKEL SILVER COPPER - NICKEL ALLOY 90-10 COPPER - NICKEL ALLOY 80-20 430 STAINLESS STEEL NICKEL, ALUMINUM, BRONZE (CA 630, 632) MONEL 400, K500 SILVER SOLDER NICKEL (PASSIVE) 60 NI- 15 CR (PASSIVE) INCONEL 600 (PASSIVE) 80 NI- 20 CR (PASSIVE) CHROME IRON (PASSIVE) 302, 303, 304, 321, 347, STAINLESS STEEL (PASSIVE) 316, 317, STAINLESS STEEL (PASSIVE) CARPENTER 20 CB-3 STAINLESS (PASSIVE), INCOLOY 825NICKEL - MOLYBDEUM - CHROMIUM - IRON ALLOY (PASSIVE) SILVER TITANIUM (PASS.) HASTELLOY C & C276 (PASSIVE), INCONEL625(PASS.) GRAPHITE ZIRCONIUM GOLD PLATINUM PROTECTED END (CATHODIC OR MOST NOBLE) |

Example of non or improper boat bonding due to incorrect zinc attachment |
|
↑ Recommend ZRD |
|