In the first two (before and after) pictures (top right), the used zinc was removed after 8 months. Although it was not reattached, it still had many useful months of life that could have been utilized. The next picture down shows an example of a Max-Prop zinc's full life utilization. Although this zinc was removed after only apx. 5 1/2 months due to extremely high salinity in the area, it was attached (used) for 95% of its useful life. Without the "attachment screw protection methodology" being utilized, this zinc would probably have been replaced after only 8 weeks of use. One edge broke off during the zinc removal due to the nature of replacing zincs underwater. Note the highest worn areas are not at the screw attachment points. The highest worn areas are at the zinc's location of the maximum material (thickness). The zinc was used the most in the middle and between the screw holes. This example demonstrates how using this methodology makes possible the longest useful life of a zinc.
After listening to feedback from companies like ZRD, PYI has a redesigned Max-Prop Zinc available (next photo down) that has an integral washer around the entire zinc at the screw holes. Electrical current is now distributed more evenly and will stop the zinc from burning through at the screws. This is shown in the third picture from the left. For users that may not have access to these special zincs or non Max-Prop applications, such as those shown in the next picture and described in the following paragraph, ZRD's tried and true methodology is unavoidable, required, and highly accepted as the only way to go.
After discovering this wonderful solution, the concept was applied to other situations. Below the max prop zinc pictures, are two pictures of a zinc installed in a Generator. Prior to the zinc having protection applied where the actual zinc connects with the threads contacting the metal to be protected, it would loose many useful months of use due to accelerated wear at the threads contact point. This would cause the zinc material to separate and fall off from the brass bolt head. The top picture shows a new zinc prior to installation and the bottom picture shows a perfectly wearing zinc after many months of use.
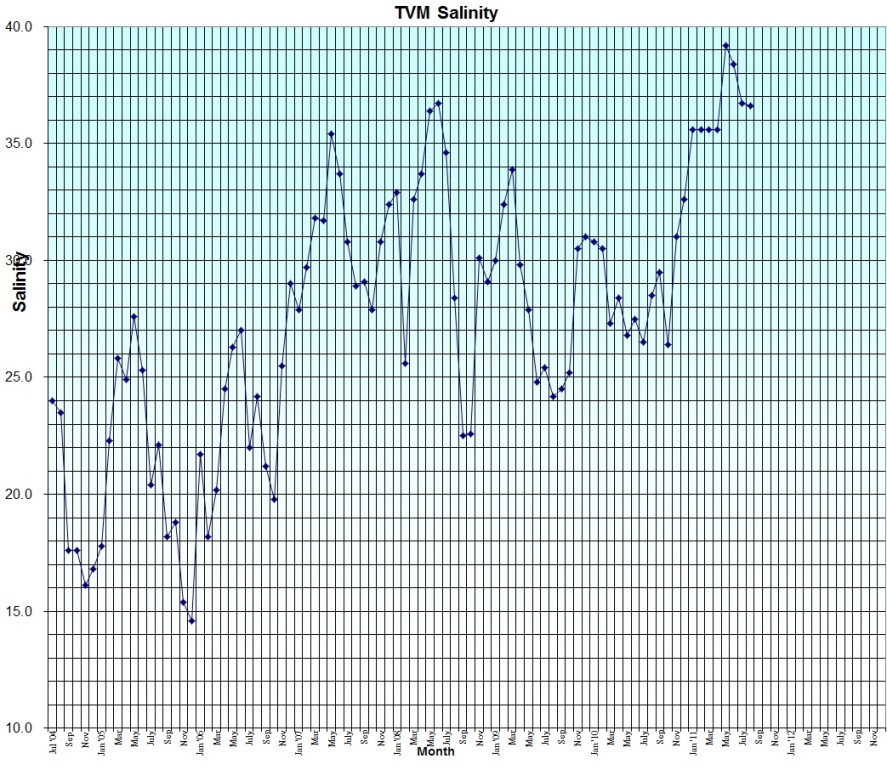
At the bottom is five years of local salinity data. The local (FL Indian River Mosquito Lagoon) environmental conditions are directly affected by the amount of rain and sunshine. Standard cycles create a normal peak of salinity (highest value) around the beginning of summer. The lowest salinity is obtained at the beginning of winter. Analysis of the data shows a clear relationship as to the causes and reasons. Starting with the low salinity at the beginning of winter, as sunshine starts to increase, water is evaporated from the lagoon area raising the salinity. Rainfall is at its lowest level adding to the 'behind the curve' scenario. Salinity continues to climb until the summer rains start in earnest around the beginning of summer. As the rainfall increases above the evaporation rate and sunlight decreases, salinity starts to decrease. The graph below shows this normal progression with various monthly bumps caused by variations in rain (sometimes, tropical systems) or sunlight (clouds). A significant note is that the trend occurs ever year, but due to the local deficit of rain in Florida over the last three years, the 'normal' low and high points of 15 and 27 have been raised to 25 and 37. There was a significant increase in rainfall in the fall of 2008, but it was short lived and appears to be a temporary abnormality in the (so far) 5 year drought.
In our opinion, there are many factors that affect zinc life and utility, but the local salinity appears to have the greatest affect on reducing zinc life. If any of the normal 'Dock Talk' items are in existence, such as a neighbor's 'hot' boat, it will typically cause zincs to 'explode' instead of just causing a premature fast decay rate like the effect of high salinity.